Molecules Are Passed Around Again and Again Within the Width
Smell is the well-nigh chemical of all the senses - simply what's the theory behind the practice?
In Short
-
Our ability to aroma comes down to unlike molecules activating different combinations of olfactory receptors
Like Proust, for whom the olfactory property of a madeleine dipped into tea recalled childhood Lord's day mornings with his aunt, most of us are familiar with the aroma of cut grass, or a piece of bluish cheese, or an amazing white wine, or Chanel No 5 perfume. Olfactory property is the about chemical of all the senses, intimately rooted in our memories. But do nosotros know how nosotros olfactory property these 'fragrances'?

Such smells tin be attributed to specific molecules, though the smell of many substances comes from a combination of many molecules. Moreover small changes in molecules can make an astonishing difference to their olfactory property.
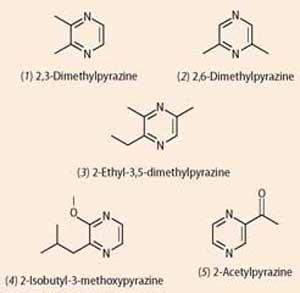
Simple alkylpyrazines like two,3-dimethylpyrazine (1) and ii,6-dimethylpyrazine (ii) are responsible for the nutty smells of roasted peanuts and baked bread; simply a small tweak, and 2-ethyl-iii,5-dimethylpyrazine (iii) is i of the 'chocolate' molecules that rounds out the roasted smell of coffee. Substituting slightly different groups to give ii-isobutyl-3-methoxypyrazine (4) gives green bell peppers their distinctive scent, which can be detected equally low as 0.002 parts per billion; and two-acetylpyrazine (5) smells of roasted popcorn.
How exercise we smell?
Scientists have been interested in how we odor molecules for many years. To be smelt, the molecules must vaporise, either as a upshot of evaporation if a liquid or sublimation if a solid (eg menthol or camphor). They are therefore fairly small molecules, with a Mr of beneath about 300 (note some pocket-sized molecules, like glucose (Mr = 180), are odourless if they are not-volatile).
Molecules that can be smelt must also at least have some solubility in h2o and be lipophilic. (Note: some volatile molecules cannot be smelled, such as HtwoO, O2, Due northtwo and, tragically, CO.)
As molecules turn down the olfactory organ, they achieve the olfactory epithelium, a region of tissue with an area of around iii-4 cm2, near the top of each nostril. Here the volatile molecules encounter odour receptors, which are covered in mucus-coated hairs or 'cilia'.
The molecules dissolve in the mucus (and thus need to have some h2o-solubility) and are carried to the receptors, by specific 'transport' proteins. When you have a cold, your sense of odor is reduced because molecules cannot attain the odor receptors.
Scientists have tried to make links between molecules and their odor using 2 different types of theory - one based on the shape of molecules and the other on their vibrational properties.
Shape theory
In 1946, Linus Pauling proposed that the scent of a molecule was determined by its shape and size. This thought was taken upwardly past R. W. Moncrieff, and developed past John E. Amoore, and was based on a 'lock-and-key' principle. Amoore suggested that there were 7 types of 'primary' odours - camphoraceous, musky, floral, pungent, ethereal, minty and putrid (other types such as almond, effluvious and aniseed were also possibilities). He hypothesised that these corresponded to different shaped receptors, which recognised different 'shaped' odorants. The overall scent was determined by the strength of binding of a molecule to the various receptors.
By the mid-1960s this theory was believed to be inadequate, and was supplanted by the 'weak shape' or odotype theory. This theory, once again based on the shape of molecules, suggested that receptors probe sections of a molecule, and that the overall odour reflects the combination of the responses from the different receptors. 1
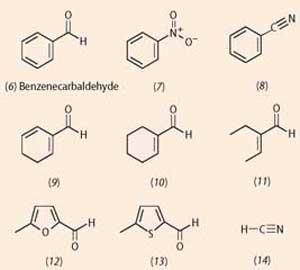
Like most processes involving recognition of molecules, odotype theory was based on a fit with a receptor, similar to enzyme-substrate interactions. Scientists looked for common structural features in molecules of a item shape. Benzenecarbaldehye (benzaldehyde,6) for example, is well known for its use in almond essence. Molecules (vii)-(xiv) take similar, simply not identical, 'almond' smells. Annotation that all these 'almond' molecules, bar one, share the structural chemical element of a multiple bond conjugated with a C=C bond (or its equivalent in a benzene ring). The exception is hydrogen cyanide, a molecule with a very different structure.
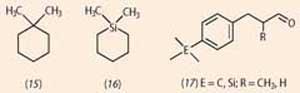
Significant smell differences have, however, been reported for molecules with like shapes. For case, one,ane-dimethylcyclohexane (15) is described as camphoraceous with a faint sweet fruity, powdery, background; the Si substituted analogue, i,1-dimethyl-1-silacyclohexane (16) has an intense chemic-light-green notation reminiscent ofcis -iii-hexenol, with a faint camphoraceous background. 2 In contrast, silicon substituted analogues of two 'lily-of-the-valley' odorants (17) accept merely subtly different smells to their entirely carbon-based analogues. In line with this, estimator modelling of the fit to the receptor binding site indicated very similar binding. three
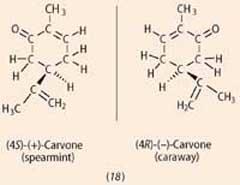
One statement more often than not given in favour of odotype theory is based on chiral molecules. While near enantiomer pairs have similar smells (to humans), a meaning minority have unlike smells, the most familiar example existence the isomers of carvone (18) (spearmint and caraway smells). 4

A'shape-based' theory explains this departure in aroma past pointing out that binding of optical isomers to chiral protein-based receptors will differ, whereas the two molecules volition have identical infrared spectra. Biophysicisist, Luca Turin suggested that ane isomer may exist bound to the receptor so that its carbonyl group is oriented unfavourably with respect to excitation, explaining the difference in olfactory property. In an experiment he added pentan-2-1 to the 'minty' carvone in a iii:2 ratio. The mixture acquired a 'caraway' odour note, and Turin suggested that this was because 'minty' carbonyl groups were now detectable by the spectroscope. 5 However, as the perfume chemist Charles Sell pointed out, 6 if the booze nonan-i-ol is added to 'minty' carvone, this mixture also acquires a 'caraway' smell, so Turin's experiment was not conclusive.
Vibrational theory
In 1938 Malcolm Dyson put forward the vibrational theory of aroma, which was developed by Robert H. Wright (1964), and has recently been modified past Turin (1996). According to Turin, receptors discover vibrational frequencies of odour molecules. Molecular vibrations, he suggests, are transmitted from olfactory receptor proteins (which effectively deport as vibrational spectrometers) via a mechanism of electron tunnelling. When the receptor site is empty, electrons are unable to tunnel beyond (traverse) the binding site, because no suitable pathway exists; when an odorant is bound, electrons can lose energy past tunnelling, through exciting an appropriate vibrational mode of the molecule. This electron flow is transmitted through a zinc ion to a G-protein, activating the receptor. 5
In favour of the vibrational model, Turin argued that the presence of certain functional groups, such as
-SH and -NO2, often gives rising to feature odours (such as 'bad egg') that appear to reflect the functional group rather than molecular shape. He suggested that the 'unique' smell character of thiols (nearly famously used by skunks) coincides with the Due south-H stretching frequencyca 2550 cm-1, a region found in few other molecules, though decaborane, B10 H14, whose B-H stretching vibrations likewise autumn in this region, has a like odor. All the same, not all thiols have this nauseating olfactory property, a prominent exception being 1-p -methene-eight-thiol, the fundamental odorant responsible for the smell of grapefruit.
Turin went on to make the post-obit predictions based on his theory:
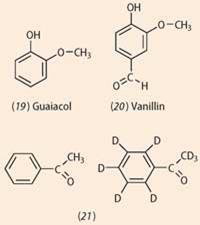
- a mixture of guaicol (19) and benzenecarbaldehyde (6) would accept a olfactory property similar to vanillin (20) because, taken together, these two molecules incorporate the same functional groups and hence vibrational frequencies, equally vanillin. Double-blind studies on human volunteers by contained researchers, 7 nonetheless, gave no show to support Turin's view;
- odd-carbon aldehydes have characteristic smells, different to the characteristic smells of even-carbon aldehydes (C8 -C12). The odd-carbon molecules odour largely waxy, with a groundwork note of citrus, while the fifty-fifty-carbon aldehydes have a mainly citrus smell, with a 'waxy' background. Independent research indicated that the chain length was the determining gene; 7
- the deuterated phenylethanone (21) has a less sweet, more solvent-like scent than phenylethanone. These molecules accept significantly dissimilar vibrational spectra just identical shapes. All the same, again independent research revealed that volunteers were not able to detect whatsoever departure. 7
Modern insight
It appears, then, that neither theory is correct. Modernistic biochemical agreement considers that the arrival of the smelt molecule activates the olfactory receptor (OR) proteins, which span membranes and incorporate vii helices (7TM receptors). Nerve impulses are sent from the receptors to specific areas of the brain responsible for olfactory property (glomeruli in the olfactory bulb). Signals from each kind of receptor cell are believed to come to the same glomerulus. The pattern of signals arriving at the brain constitutes the 'smell' of the molecule perceived by the brain.
In 1991, Richard Axel and Linda Buck reported on the family unit of genes coding for odorant receptors. 8 (This work was recognised by the honour of the Nobel Prize in physiology or medicine in 2004.)
Buck and her squad went on to study 9 the interaction betwixt private odorant receptors and Cfour -C9 aliphatic odorants with different functional groups (eg alcohol, carboxylic acid), and found that:
- each olfactory receptor (OR) can recognise many odorants;
- each odorant can exist detected by several different ORs;
- unlike odorants can exist detected by different OR combinations, leading to stardom by the encephalon.
The researchers concluded that it is not a example of i odorant for one receptor but rather that different molecules activate a different combination of ORs, and so sends a different signal to the encephalon. Given that there are around 340 unlike agile ORs in humans (in contrast to around 1000 in mice and rats), this system can discriminate between large numbers of odorants. This explains why though there are just a few hundred different receptors, humans can recognise 10,000 or more different odours.
Even small-scale changes in the odorant molecule, such as using octan-1-ol instead of octanoic acid, produces a change in the combination of ORs detecting information technology. The researchers also found that a change in odorant concentration may alter its receptor code. This explains why sometimes a substance has different smells at different concentrations; for example, dimethyl sulfide. Other researchers have shown that some neurons in the olfactory cortex merely respond when two odorants are present and not when each component is separately present, explaining why mixtures tin have smells that are not merely the sum of the component molecules. 10
Humans devote a smaller fraction of the olfactory epithelium to olfactory neurons compared with other animals, where the density of receptors is also much greater. Dogs, for instance, can distinguish breath samples of lung and breast cancer patients, xi and urine samples from bladder cancer patients, 12 evidently because of the presence of infinitesimal quantities of volatile organic molecules, acting as biochemical markers (though the molecules responsible accept not yet been identified). In a recent experiment olfactory receptors were expressed in yeast cells and fastened to a sensor chip, and shown to go on both the selectivity and sensitivity of the natural receptor. This could class the basis of bio-electronic sensors that could find odorants in urine, for example, that human activity as markers for the presence of a disease, drugs, or explosives. 13 This could add an exciting new dimension to the chemistry of smell.
Dr Simon Cotton is a chemistry teacher at Uppingham School, Uppingham, Rutland LE15 9QE.
Further Reading
J. M. Berg, J. 50. Tymoczko and Fifty. Stryer,Biochemistry, fifth edn, pp 897-903. New York: W. H. Freeman, 2002.
L. B. Buck,Angew. Chem. Int. Edn. Engl., 2005, 44 , 6128.
Linda Cadet's Nobel lecture, bachelor online
C. S. Sell,Chem. Br ., March 1997, 39;Angew. Chem. Int. Edn Engl., 2006, 45, 6254.
D. A. Wilson and R. J. Stevenson,Learning to smell. Baltimore: The John Hopkins University, 2006.
50. Turin,The undercover of scent. London: Faber and Faber, 2006.
Website on olfaction produced by Tim Jacob of the School of Biosciences at Cardiff University
Related Links
- Linda Buck Nobel lecture
- Tim Jacob Research and teaching information
lombardibothe1936.blogspot.com
Source: https://edu.rsc.org/feature/if-it-smells-its-chemistry/2020168.article
0 Response to "Molecules Are Passed Around Again and Again Within the Width"
Post a Comment